|
Size: 5128
Comment:
|
Size: 4653
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 1: | Line 1: |
| /!\ PAGE UNDER CONSTRUCTION / NOT PUBLIC YET |
#acl All:read |
| Line 8: | Line 7: |
| == Outline == | = Outline = |
| Line 13: | Line 12: |
| * You need to have Cytoscape installed : minimally 2.6.3 must be installed but preferable to have the latest version of cytoscape * Install the Enrichment Map plugin from the Cytoscape plugin manager. If you install it manually (e.g. if you need to install a new version that doesn't happen to be in the plugin manager yet), then it must be in the Cytoscape-[Version#]/plugins folder * You need to download the test data: [[attachment:DavidTutorial.zip]] |
* You need to have Cytoscape installed : minimally 2.6.3 must be installed but preferable to have the latest version of Cytoscape (e.g. version 3.1) * Install the Enrichment Map plugin from the Cytoscape plugin manager. If you install it manually (e.g. if you need to install a new version that doesn't happen to be in the plugin manager yet), then it must be in the Cytoscape-[Version#]/plugins folder --> '''For Cytoscape 2.8''' * Install the Enrichment Map App from the Cytoscape App manager in cytoscape 3. --> '''For Cytoscape 3.''' |
| Line 17: | Line 16: |
| Description of the tutorial files contained in the gProfilerTutorial folder: * 12hr_topgenes.txt : List of top genes expressed in Estrogen dataset at 12hr - Official Gene Symbol. * 24hr_topgenes.txt : List of top genes expressed in Estrogen dataset at 24hr - Official Gene Symbol. * 12hr_David_Output.txt : Estrogen treatment - 12hr DAVID result chart - [[./WhereToGetDavidChart|Screen shot of where to get DAVID output chart]] * 24hr_David_Output.txt : Estrogen treatment - 24hr DAVID result chart * Estrogen_expression_file.txt: Expression File - Estrogen treatment, Official Gene Name as key. |
= Instructions = == Step 1: Generate g:Profiler output files == * You need to download the test data: [[attachment:gProfilerTutorial.zip]] * Description of the tutorial files contained in the gProfilerTutorial folder: * 12hr_topgenes.txt : List of top genes expressed in Estrogen dataset at 12hr - Official Gene Symbol. * 24hr_topgenes.txt : List of top genes expressed in Estrogen dataset at 24hr - Official Gene Symbol. |
| Line 25: | Line 25: |
| == Instructions == === Step 1: Generate g:Profiler output files === 1. Go to g:Profiler website - [[http://biit.cs.ut.ee/gprofiler/]] 1. Select and copy all genes in the tutorial file 12hr_topgenes.txt in the 'Query' box 1. In 'Options', check 'Significant only', 'No electronic GO annotations' 1. Set the 'Output type' to 'Generic !EnrichmentMap' 1. Show advanced options 1. Set 'Max size of functional category' to 500 1. Set 'Significance threshold' to Benjamini-Hochberg FDR 1. Click on 'g:Profile!' to run the analysis 1. Download g:Profiler data as gmt: "name" 1. Download the result file: " Download data in Generic Enrichment Map (GEM) format" |
1. '''Go to g:Profiler website''' - [[http://biit.cs.ut.ee/gprofiler/]] 1. Select and copy all genes in the tutorial file 12hr_topgenes.txt in the '''Query''' box. /!\ make sure that your list contains only official gene symbol (HUGO) 1. In '''Options''', check '''Significant only''', '''No electronic GO annotations''' 1. Set the '''Output type''' to '''Generic !EnrichmentMap''' 1. '''Show advanced options''' 1. Set '''Min and Max size of functional category''' to '''3''' and '''500''' respectively. 1. Select '''2''' for '''Size of Q&T''' 1. On the right panel, choose the '''Gene Ontology Biological process''' and '''Kegg''' and '''Reactome''' 1. Set '''Significance threshold''' to '''Benjamini-Hochberg FDR''' 1. Click on '''g:Profile!''' to run the analysis a. (Note) - if some of your identifiers in your query have multiple mappings in g:Profiler! by default they get excluded. If this happens you will see the following above the g:Profiler! results: {{attachment:gProfiler_warning_screenshot.png}} a. Click on above link to manually map each gene to its correct annotation {{attachment:gProfiler_warning_expanded_screenshot.png}} a. click on '''Resubmit query''' to update your results with the specified mappings. 1. Download g:Profiler data as gmt: '''name''' Note: you will have to unzip the folder 1. Download the result file: ''' Download data in Generic Enrichment Map (GEM) format''' |
| Line 40: | Line 45: |
| {{attachment:gProfiler_screenshot.tiff}} | {{attachment:gProfiler_screenshot.png}} |
| Line 42: | Line 47: |
| === Step 2: Generate Enrichment Map with g:Profiler Output === {{attachment:David_inputpanel.png|Screenshot David Input Panel|align="right"}} 1. Open Cytoscape 1. Click on Plugins / Enrichment Maps / Load Enrichment Results 1. Make sure the Analysis Type is set to DAVID/BiNGO 1. Please select the following files by clicking on the respective (...) button and selecting the file in the Dialog: * '''NO GMT file is required for DAVID Analysis''' * Dataset 1 / Expression: `Estrogen_expression_file.txt` (OPTIONAL) * Dataset 1 / Enrichments: `12hr_David_Output.txt` * Click on "''Dataset 2 {{attachment:arrow_collapsed.gif}}''" to expand the panel * Dataset 2 / Expression: ''leave empty'' * Dataset 2 / Enrichments 1: `24hr_David_Output.txt` (OPTIONAL) 1. Tune Parameters * P-value cut-off `0.001` * Q-value cut-off `0.05` * Check Overlap Coefficient * Overlap coefficient cut-off `0.6` 1. Build Enrichment Map 1. Go to View, and activate Show Graphics Details '''NOTE:There are multiple values in DAVID that can be used for the Q-value in EM including Bonferroni, Benjamini, and FDR. In EM we use the Benjamini as the Q-value.''' <<BR>><<BR>><<BR>><<BR>><<BR>><<BR>><<BR>><<BR>> === Step 3: Examining Results === {{attachment:David_EM.png|DAVID EM Result}}<<BR>> |
<<BR>> {{attachment:gProfiler_inputpanel.png|Screenshot gProfilet EM Panel|align="right"}} * Link to a step by step tutorial: [[attachment:gProfiler_step_by_step.pdf]] == Step 2: Generate Enrichment Map with g:Profiler Output == <<BR>><<BR>> * g:Profiler output files [[attachment:gProfiler_EM.zip]] <<BR>><<BR>> *1. '''Open Cytoscape''' <<BR>> *2. In the menu bar, locate the '''App''' tab and then select --> '''!EnrichmentMap''' --> '''Create Enrichment Map''' <<BR>> *3. Make sure the Analysis Type is set to '''generic(ex:gProfiler)''' <<BR>> *4. '''Please select the following files by clicking on the respective (...) button and selecting the file in the Dialog''': * GMT / '''hsapiens.NAME.gmt''' * Dataset 1 / Enrichments: '''gProfiler_results_12hr.txt''' <<BR>> *5. '''Tune Parameters''' * P-value cut-off '''1''' * Q-value cut-off '''1''' * Overlap Coefficient cut-off '''0.5''' <<BR>> *6. Click on the '''Build''' radio button at the bottom of the panel to create the Enrichment Map <<BR>> *7. In the menu bar, Go to '''View''', and activate '''Show Graphics Details''' <<BR>><<BR>> == Step 3: Examining Results == {{attachment:gProfiler_EM.png|gProfiler EM Result}}<<BR>> |
| Line 66: | Line 81: |
| 1. Node (inner circle) size corresponds to the number of genes in dataset 1 within the geneset 1. Node border (outer circle) size corresponds to the number of genes in dataset 2 within the geneset 1. Colour of the node (inner circle) and border(outer circle) corresponds to the significance of the geneset for dataset 1 and dataset 2, respectively. 1. Edge size corresponds to the number of genes that overlap between the two connected genesets. Green edges correspond to both datasets when it is the only colour edge. When there are two different edge colours, green corresponds to dataset 1 and blue corresponds to dataset 2. * '''NOTE''': if you are using two enrichment sets you will see two different colours of edges in the enrichment map. When the set of genes in the two datasets are different (for example, when you are comparing two different species or when you are comparing results from two different platforms) the overlaps are computed for each dataset separately as there is a different set of genes that the enrichments were calculated on. In this case, since the enrichments were reduced to only a subset of most differentially expressed at each time point the set of genes the enrichments are calculated on are different and overlap are calculated for each set separately. |
1. Node size corresponds to the number of genes in dataset 1 within the geneset 1. Colour of the node corresponds to the significance of the geneset for dataset 1. 1. Edge size corresponds to the number of genes that overlap between two connected genesets. |
Enrichment Map g:Profiler Tutorial
Contents
Outline
This quick tutorial will guide you through the generation of an Enrichment Map for an analysis performed using g:Profiler (Functional Profiling of Gene List from large-scale experiments)
To run this tutorial:
- You need to have Cytoscape installed : minimally 2.6.3 must be installed but preferable to have the latest version of Cytoscape (e.g. version 3.1)
Install the Enrichment Map plugin from the Cytoscape plugin manager. If you install it manually (e.g. if you need to install a new version that doesn't happen to be in the plugin manager yet), then it must be in the Cytoscape-[Version#]/plugins folder --> For Cytoscape 2.8
Install the Enrichment Map App from the Cytoscape App manager in cytoscape 3. --> For Cytoscape 3.
Instructions
Step 1: Generate g:Profiler output files
You need to download the test data: gProfilerTutorial.zip
- Description of the tutorial files contained in the gProfilerTutorial folder:
- 12hr_topgenes.txt : List of top genes expressed in Estrogen dataset at 12hr - Official Gene Symbol.
- 24hr_topgenes.txt : List of top genes expressed in Estrogen dataset at 24hr - Official Gene Symbol.
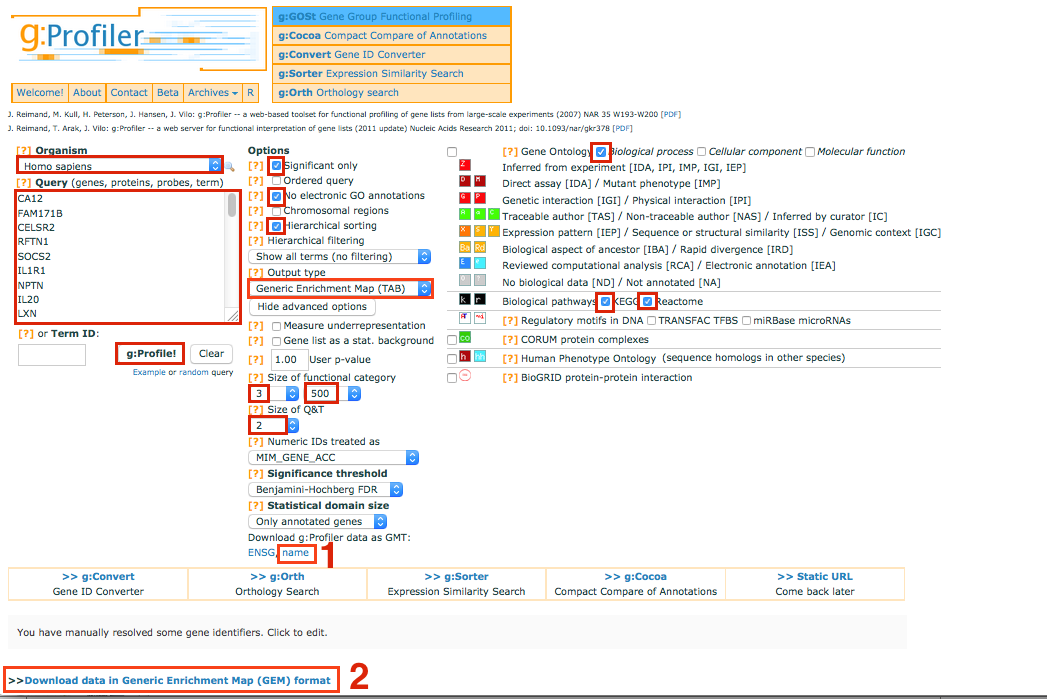
Go to g:Profiler website - http://biit.cs.ut.ee/gprofiler/
Select and copy all genes in the tutorial file 12hr_topgenes.txt in the Query box.
 make sure that your list contains only official gene symbol (HUGO)
make sure that your list contains only official gene symbol (HUGO) In Options, check Significant only, No electronic GO annotations
Set the Output type to Generic EnrichmentMap
Show advanced options
Set Min and Max size of functional category to 3 and 500 respectively.
Select 2 for Size of Q&T
On the right panel, choose the Gene Ontology Biological process and Kegg and Reactome
Set Significance threshold to Benjamini-Hochberg FDR
Click on g:Profile! to run the analysis
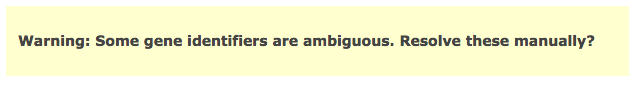
- (Note) - if some of your identifiers in your query have multiple mappings in g:Profiler! by default they get excluded. If this happens you will see the following above the g:Profiler! results:
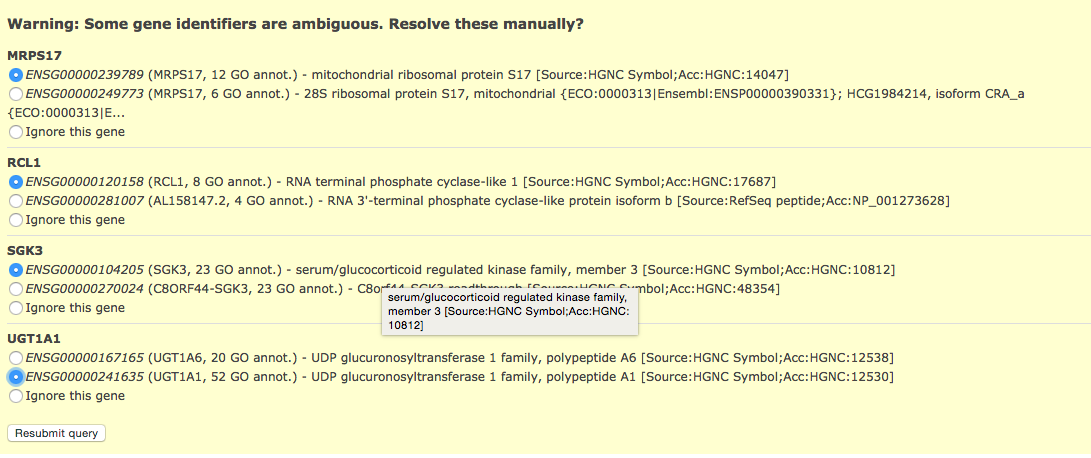
- Click on above link to manually map each gene to its correct annotation
click on Resubmit query to update your results with the specified mappings.
- (Note) - if some of your identifiers in your query have multiple mappings in g:Profiler! by default they get excluded. If this happens you will see the following above the g:Profiler! results:
Download g:Profiler data as gmt: name Note: you will have to unzip the folder
Download the result file: Download data in Generic Enrichment Map (GEM) format
Note: repeat these steps for the 24hrs time-point and the file 24hr_topgenes.txt
Link to a step by step tutorial: gProfiler_step_by_step.pdf
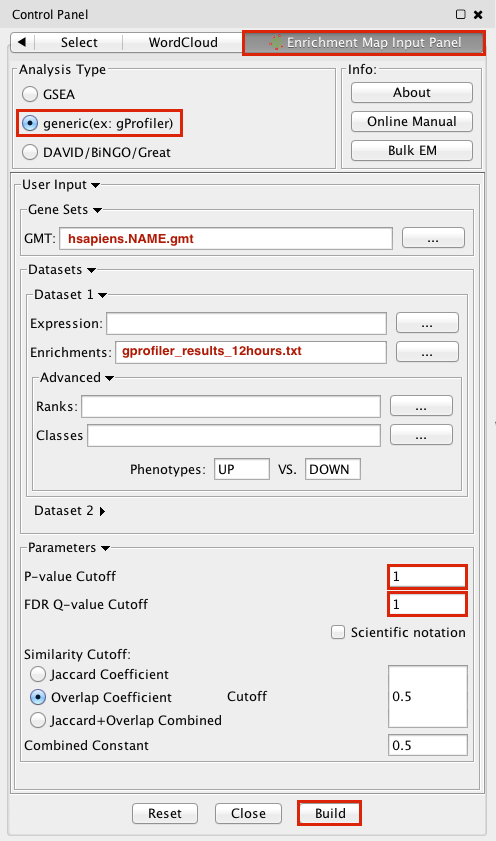
Step 2: Generate Enrichment Map with g:Profiler Output
g:Profiler output files gProfiler_EM.zip
1. Open Cytoscape
2. In the menu bar, locate the App tab and then select --> EnrichmentMap --> Create Enrichment Map
3. Make sure the Analysis Type is set to generic(ex:gProfiler)
4. Please select the following files by clicking on the respective (...) button and selecting the file in the Dialog:
GMT / hsapiens.NAME.gmt
Dataset 1 / Enrichments: gProfiler_results_12hr.txt
5. Tune Parameters
P-value cut-off 1
Q-value cut-off 1
Overlap Coefficient cut-off 0.5
6. Click on the Build radio button at the bottom of the panel to create the Enrichment Map
7. In the menu bar, Go to View, and activate Show Graphics Details
Step 3: Examining Results
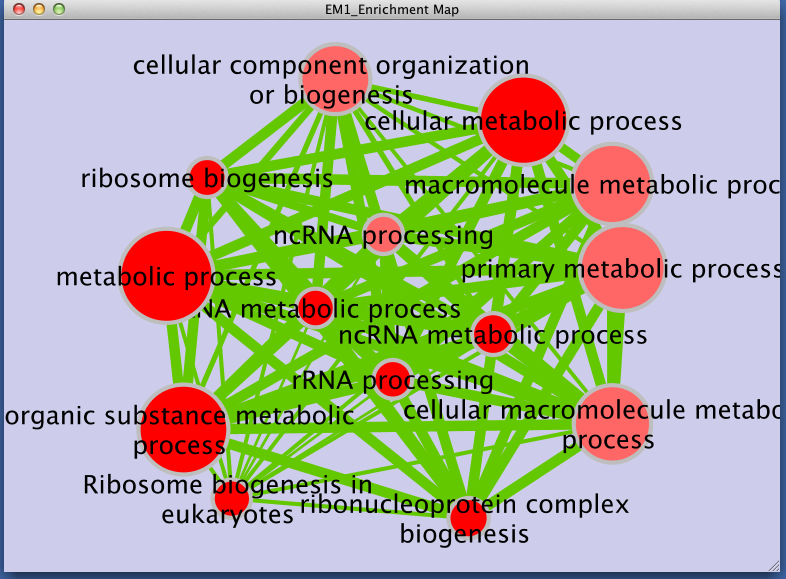
Legend:
- Node size corresponds to the number of genes in dataset 1 within the geneset
- Colour of the node corresponds to the significance of the geneset for dataset 1.
- Edge size corresponds to the number of genes that overlap between two connected genesets.